Covid: People aged 50-54 invited for jab in England
 image copyrightGetty Images
image copyrightGetty ImagesPeople aged 50 and over in England are now being invited to book their appointment for a Covid vaccine.
This is the final group on the priority list, which covers 99% at high risk of dying from Covid-19.
Everyone in the top nine priority groups should be offered a second dose by mid-July, up to 12 weeks after the first.
Nearly half of the UK adult population has had one vaccine dose - more than 24 million people.
Around 1.6 million people have also had a second dose.
In Northern Ireland and some areas of Scotland, the over-50s are already being offered a Covid vaccine. Parts of England may already have started offering it to this age group too, with Wales targeting an offer of one dose to all over-50s by mid-April.
In total, the number of people who have had one vaccine dose is:
- More than 21 million in England
- About 1.9 million in Scotland
- Just over one million in Wales
- More than 630,000 in Northern Ireland
The number of vaccines given this week in the UK is expected to top four million - nearly double what has been achieved per week recently - thanks to a large shipment of the Oxford-AstraZeneca vaccine from India.
This could mean all those aged 50 and over will be offered one dose by the end of March, putting the NHS rollout two weeks ahead of schedule.
After that, the rest of the adult population will be vaccinated, with people prioritised by age.
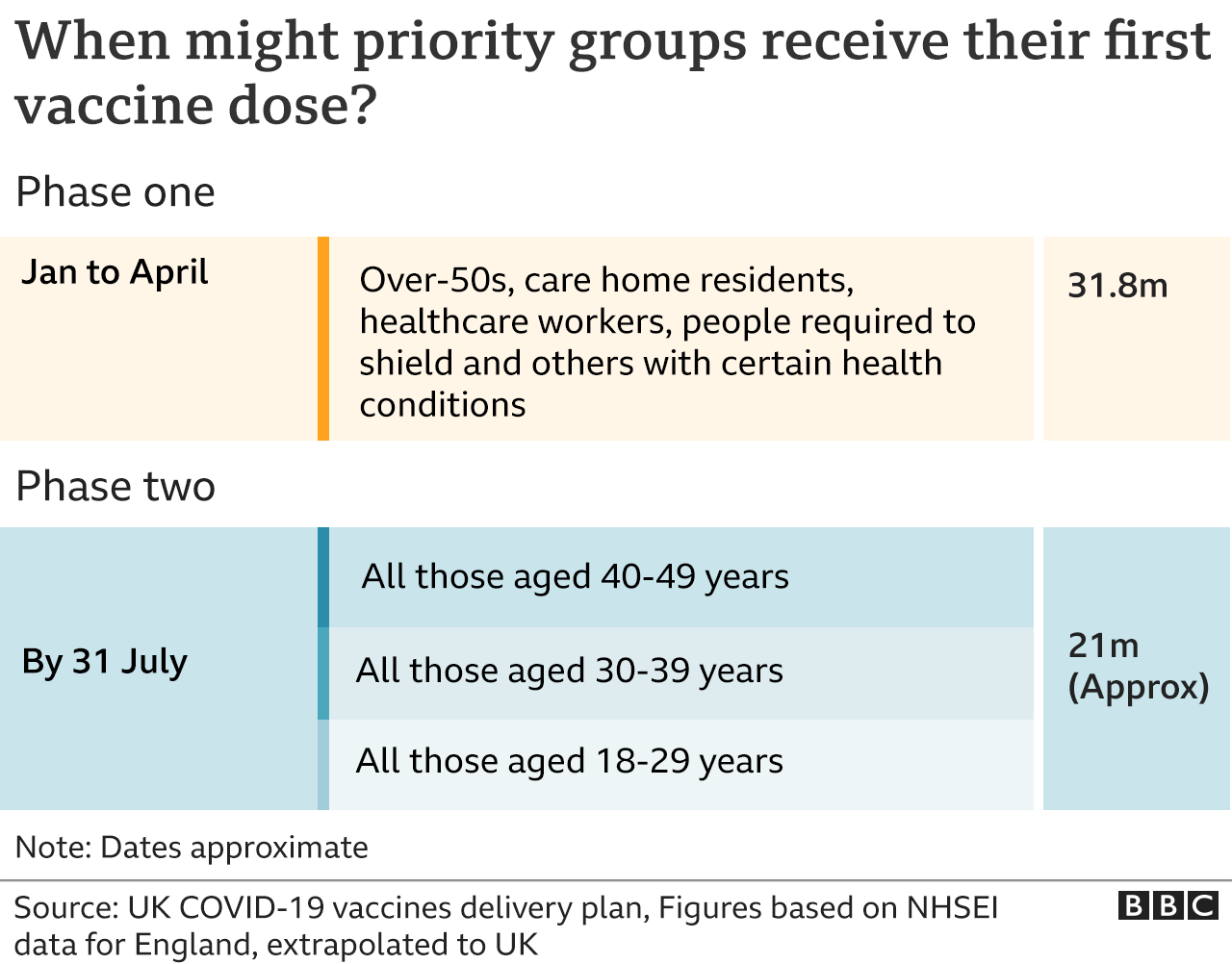
All adults in the UK are expected to be offered their first dose of a coronavirus vaccine by the end of July.
It is 100 days since Margaret Keenan became the first person in the UK to be vaccinated against Covid outside of clinical trials.
Health Secretary Matt Hancock said: "I'm determined no-one should miss out on the chance to protect themselves and urge everyone who is eligible to come forward."
Dr Nikki Kanani, GP and NHS England's primary care director, said vaccine supplies would go up and down over the next few months linked to manufacturers' ability to supply their jabs.
"But this week and next we have larger supplies, so we want anyone in the top priority groups - people aged 50 and older as well as those working in health and care and anyone with an underlying health condition - to come forward soon to protect themselves and their loved ones," she said.
Text invitations
Two million text messages are being sent out to those aged 50-54 in England with a link allowing people to book an appointment through the national booking service website.
People who cannot go online can call the service on 119.
The expansion of the Covid vaccine rollout comes after government reassurance that the Oxford-AstraZeneca vaccine - one of two being given to people in the UK - was safe, after 13 countries paused their use of it.


Expect a surge in vaccinations in the next week or so. Extra supplies of the AstraZeneca vaccine have arrived from abroad to supplement existing stocks.
The target to offer a vaccine to all those aged 50 and over by mid-April will almost certainly be met ahead of schedule. It will then be the turn of younger adults.
The progress means the UK is easing restrictions as parts of Europe face new waves of infection.
Concerns have been raised about safety of the AstraZeneca jab - the European regulator is investigating cases of blood clots, focusing on a rare type that affects the brain.
Evidence from 10 million doses given in the UK show there have been three cases - none fatal.
This is not above the level you would expect to happen normally, suggesting coincidence rather than cause.
In Germany the numbers are higher - but because these events are so rare, it's easy for a couple of cases to raise alarm.
It's understandable they are being investigated, but what has baffled experts is why some nations have paused rollout in the meantime.
After all, this vaccine saves lives. That's why authorities here say it's safe and even the European regulator says vaccination should continue.

The EU's regulator, the European Medicines Agency (EMA), is expected to release the findings of its investigation into cases of rare blood clots in a tiny number of vaccinated people on Thursday.
But the UK medicines regulator, the MHRA, and Downing Street has said there is no evidence to suggest the Oxford-AstraZeneca vaccine is linked to the clots.
Mr Hancock urged people to "listen to the regulators" and to "get the jab" as soon as they got the opportunity.
A vaccine safety panel of the World Health Organization said it was "carefully assessing the latest available data" for the vaccine but, in the meantime, recommended that vaccinations should continue.



No comments